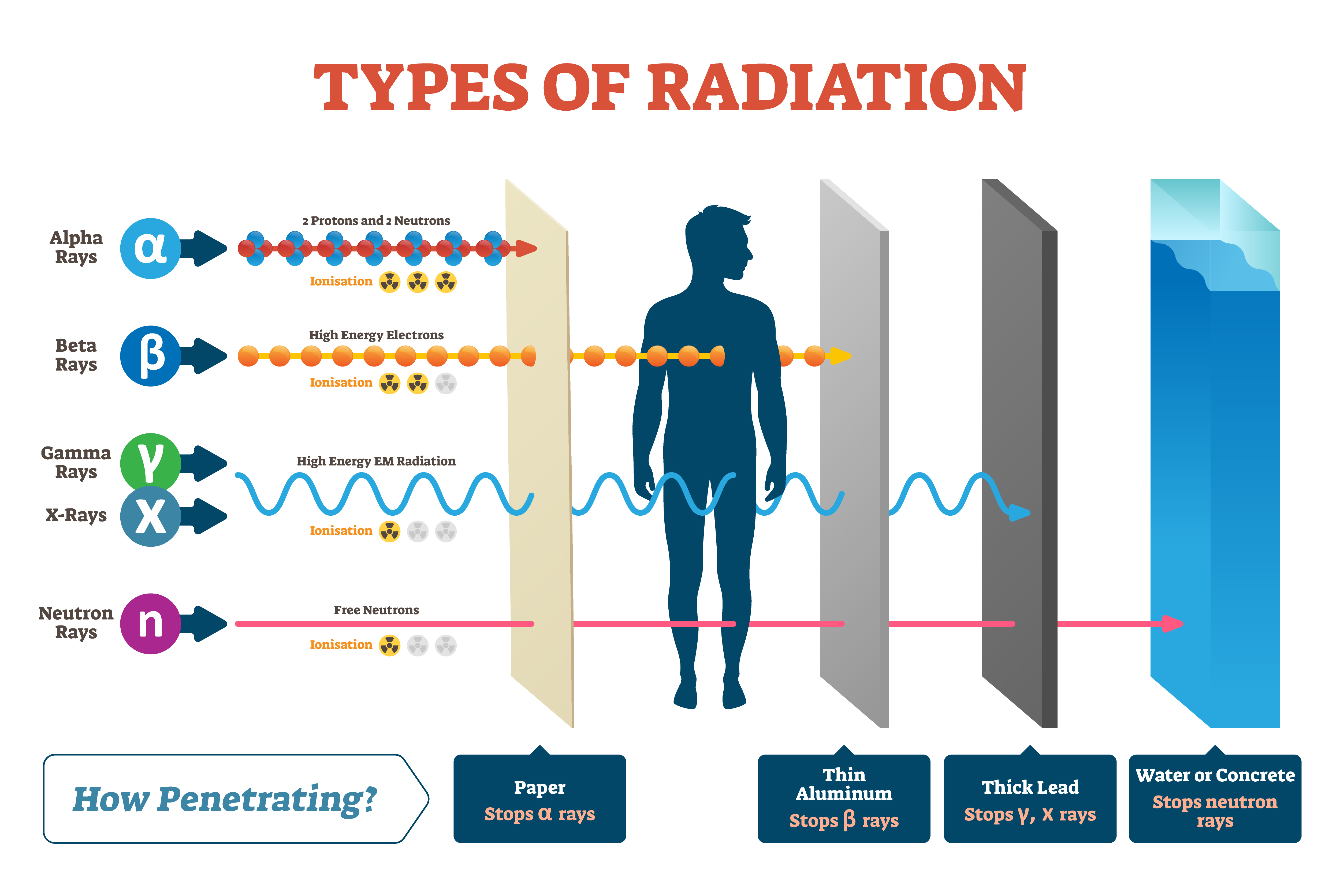
Isotopes
Radioisotopes are defined as atoms that contain an unstable combination of neutrons and protons, or excess energy in their nucleus. Atoms with an unstable nucleus regain stability by shedding excess particles and energy in the form of radiation (radioactive decay)
List of isotopes used at The Faculty of Medicine (see links for each isotope)
|
Isotope |
Radioactive decay |
Source |
Half-life Years (y), days (d) hours (h) |
Application |
|---|---|---|---|---|
|
Beta |
Sealed equipment (BioBeam) |
30.17 y |
Label cells, mice BioBeam equipment |
|
|
Gamma |
Radiotracer (PET machine) |
1.83 h |
Nuclear medicine |
|
|
Gamma |
Radiotracer (PET machine) |
6 h |
Nuclear medicine |
|
|
Alpha |
Liquid Sealed needle |
3.6 d |
Cell research |
|
|
Beta |
Liquid |
87.9 d |
Protein labeling |
|
|
Beta |
Liquid |
14.3 d |
Nucleic acid labeling |
|
|
Beta |
Liquid |
12.3 y |
Label organic molecules; drug discovery |
|
|
Beta |
Liquid |
162.6 y |
Calcium metabolism |
|
|
Beta |
Liquid |
5730 y |
Radiocarbon dating |
|
|
Alpha |
Solid |
1.91 y |
Cell research |
|
|
Alpha |
Solid |
432.7 y |
Cell research |
- Isotope Half-life is the time required for one-half of the radioactive atoms in a sample to decay or desintegrate, how fast radioactive material decays
- Appropriate decay waste is 10 half-lives (0.1%)